Lawsuits involving the birth control shots are troubling, and victims suffering any injuries should contact our Kentucky Depo-Provera lawsuit attorneys. Many people throughout the United States and right here in Kentucky rely on various different methods of birth control for family planning. According to statistics from the Centers for Disease Control and Prevention (CDC), approximately 65% of all women aged between 15 and 49 years old are using some form of contraception. Although there are many different types of birth control, one of the easiest and non-permanent options offered on the market are birth control shots. Also known as a contraceptive injection, roughly 8% women worldwide and 3-4% of women in the United States are currently relying on these birth control shots. In addition, approximately 25% of women have used a birth control shot at one time, including Depo-Provera.
Unfortunately, birth control shots like Depo-Provera may be linked to an increased risk for a patient developing brain tumors, up to a 5.6-fold increase or 560% increase, specifically intracranial meningiomas. Indeed, more and more women who have been diagnosed with brain tumors or other side effects are reporting use of birth control shots which may be a causal link, resulting in lawsuits throughout the United States.
If you or a loved one had taken any type of birth control injectable, especially Depo-Provera, and later developed any type of cancer including a brain tumor, all the Maze Law Offices in Kentucky. Our experienced Depo shot lawsuit attorneys are happy to meet with you and your family to discuss what your legal options may be, including whether you and your family may be entitled to compensation for personal injuries, lost wages, medical bills, and other damages. To learn more about your rights under Kentucky and federal law, schedule your free case consultation.
Developing cancer or brain tumors from a contraceptive should never happen, especially a contraceptive such as a birth control shot which is required to go through rigorous testing and analysis before being used. That’s because these are powerful drugs that are being used in your body to alter your hormones and how your reproductive system functions and multiple different levels.
However, medroxyprogesterone acetate injections have been shown to result in a 5.56-fold increased risk of brain tumors for patients who used it. Since Depo-Provera uses this active ingredient, contact the Maze Law Offices for help and to learn more about your rights.
Birth control shots are injectable contraceptives that contain hormones to prevent pregnancy. These shots work by releasing synthetic hormones into the bloodstream that suppress ovulation, thicken cervical mucus to block sperm, and thin the uterine lining to prevent implantation of an embryo/fertilized egg. This combination makes it improbable that a woman will get pregnant because creating mechanisms to stop a pregnancy at different points in the process (i.e., preventing the egg from being released, if it is realized by preventing fertilization, and if there is fertilization to prevent implantation).
The most common type of birth control shot is administered once every three months, providing long-acting but reversible contraception that does not require surgery or even a procedure to implant or remove. There are other shots that are every month. Sometimes the shots will be given in weekly intervals that increase in strength until they become a full dose and become fully effective. When administered correctly, injectable contraceptives are approximately 96% to 99% effective at preventing pregnancy.
Depo-Provera (depot medroxyprogesterone acetate or DMPA) is the brand name for a specific type of injectable contraceptive that contains a synthetic form of the hormone progesterone. Manufactured by Pfizer, it has been FDA-approved since 1992 and is administered as an intramuscular injection every 12-13 weeks. The shot delivers 150mg of medroxyprogesterone acetate, which maintains a consistent hormone level in the body to prevent pregnancy. While effective for contraception, Depo-Provera has been associated with various side effects, including changes in menstrual bleeding, weight gain, and potential bone density loss.
There is another smaller dose called Depo-SubQ Provera 104, which is injected just beneath the skin instead of deep into the muscle. It has similar benefits and risks, but may work differently for certain people and, therefore, may or may not be as effective.
Recent allegations have raised concerns about a potential link between long-term Depo-Provera use and the development of meningiomas, which are typically benign brain tumors that form in the meninges (the protective membranes covering the brain and spinal cord). The synthetic progesterone in Depo-Provera may stimulate the growth of these tumors, as meningiomas often have progesterone receptors.
Some research suggests that prolonged exposure to high doses of synthetic progesterone could increase the risk of meningioma development, particularly in susceptible individuals. This potential association has led to increased scrutiny of the medication’s long-term safety profile and has become a focus of pharmaceutical litigation in both individual and potential class action lawsuits.
Indeed, when the FDA gave final approval, some government research literature indicates concerns over the safety of this shot. In fact, it was not immediately approved by the FDA despite other countries approving it because of a concern of breast tumors and cervical cancer in animals. Although the World Health Organization (WHO) debunked those claims, at least relating to cervical cancer and ovarian cancer, that did not dispel the claim that there was an insignificant increased risk of breast cancer found in younger women who took it.
The risks involving Depo-Provera have been significant for many reasons. Indeed, this product has had scrutiny for many issues that have resulted in legal troubles for more than just birth control, which is evolving right now. These other issues show a pattern of neglect in failing to warn patients and users.
Depo-Provera (medroxyprogesterone acetate), manufactured by Pfizer, is facing increasing litigation regarding alleged adverse health effects, particularly focusing on claims of bone density loss and osteoporosis in long-term users. Although originally FDA approved in 1992 with some concerns, as noted above, in 2004, the FDA required Pfizer to add a black box warning regarding bone density loss, particularly concerning for adolescent users. It did not address any other allegations of cancer or brain tumors.
That warning was prompted by research found that Depo-Provera could cause bone loss and osteopenia. This has been the subject of other lawsuits, including in Canada, which had changed the warning labels to indicate that when it was originally approved. Specifically, in May 2008, plaintiffs commenced a lawsuit against Pfizer regarding the birth control shot Depo-Provera, which was subsequently certified as a class action lawsuit by a court in Quebec. The lawsuit alleged, among other things, that Pfizer knew or should have known about the increased risk of bone loss caused by its injectable birth control shot. The matter proceeded until 2010, when a proposed settlement was reached, and the court approved it in 2021 and was over $2 million for victims and insurance carriers.
As a result, some lawsuits after the warnings were implemented in the FDA approval had been dismissed for bone loss, including the dismissal of a lawsuit of a young woman who had used the shot for many years before having a cautionary scan. There have been other subsequent lawsuits relating to bone loss which have had mixed results.
But what this does show is that Depo-Provera may have known or should have known about the risk of bone loss, but did nothing about it until there was overwhelming literature and research about it. Unfortunately, individuals who tried to sue after the warnings were unsuccessful. Part of this was because they could not prove that they did not have bone loss prior to taking the drug – although they were in their 20s and 30s and therefore such advanced osteopenia was unlikely.
Lightning can strike twice, and brain tumors have been linked to Depo-Provera in some more recent research. Indeed, if the bone loss lawsuit and settlement is any indicator, it is likely that brain tumor lawsuits are going to go as well if not better – especially given the horrific nature of a brain tumor. In fact, studies of medical malpractice lawsuits involving a misdiagnosed or delayed diagnosis of a meningiomas between 1985 and 2020 have revealed the average payout to be $3.4 million in a verdict, or almost $870,000 in a settlement, per case/plaintiff. Now there is a big difference between a medical malpractice payout for an individual plaintiff where a doctor has affirmatively caused a medical error and a class action lawsuit for a drug’s dangerous side effects, but the truth is that this is a very serious claim and could be in the six- or seven-figures for plaintiffs who have had serious injuries from these brain tumors.
Despite some troubling allegations relating to brain tumors, there has still been no changes by the FDA or Pfizer related to Depo-Provera. There have been more studies initiated, by the findings are yet to be well-published – likely due to the lawsuits that are ongoing at this time. As more and more private lawsuits are starting to be filed in 2024, there will be more opportunities for victims and their families to obtain compensation for brain tumors due to this birth control shot. Indeed, families should begin to call our experienced Depo-Provera lawsuit attorneys in Kentucky now to avoid being left off from possible compensation.
The most recent lawsuit against Pfizer and Depo-Provera to make the news is by Kristina Schmidt out of California. She is a 37-year-old woman who used Depo-Provera birth control injections for 17 years, was diagnosed with a brain tumor in June 2022. She has filed a lawsuit against Pfizer, claiming that the contraceptive’s active ingredient, medroxyprogesterone acetate caused her tumor. Schmidt experienced severe symptoms including headaches, dizziness, and vertigo, ultimately requiring complex brain surgery that involved removing irregular tissue and replacing it with bovine pericardium.
The lawsuit alleges that Pfizer failed to provide adequate warnings about the risks associated with Depo-Provera, despite evidence suggesting that MPA can promote abnormal cell growth leading to tumors. This claim is supported by the March 2024 BMJ study noted below, indicating that women using the injection for over a year face a 5.6-fold increased risk of developing meningiomas. The medication, FDA-approved in 1992, has been marketed as a safe and effective contraceptive method and was only amended once for a bone loss black box, as noted above by the lawsuit in Canada.
Attorneys nationwide like our Depo-Provera lawyers in Kentucky are now reviewing Depo-Provera brain tumor cases against Pfizer on a contingency fee basis, representing clients who have developed meningioma brain tumors after using the contraceptive injection. Schmidt’s lawsuit seeks compensation for physical and emotional distress, ongoing medical issues, and diminished quality of life, while alleging inadequate testing, flawed design, misrepresentation, fraud, and breach of warranty by Pfizer.
Other lawsuits are now starting to be filed against Pfizer for hiding the links between brain tumors and the active ingredients. In the UK, Pfizer has now started with a new black box warning for intracranial meningiomas due to the active ingredient. This has not started in the United States.
New Developments in Brain Tumor Lawsuits: British Medical Journal Study Published in March 2024
Following some lawsuits in other countries and the start of them in the United States, the British Medical Journal (BMJ) published the results of a comprehensive study that had some very troubling results. Specifically, the study investigated the association between various progestogens and the risk of intracranial meningioma requiring surgery, analyzing data from the French national health system between 2009 and 2018. The research included 18,061 women who underwent meningioma surgery and 90,305 matched controls, examining multiple progestogens with different routes of administration (oral, percutaneous, intravaginal, intramuscular, and intrauterine). The study found no significant increased risk with progesterone, dydrogesterone, spironolactone, or levonorgestrel intrauterine systems. These are ingredients that are not found in Depo-Provera.
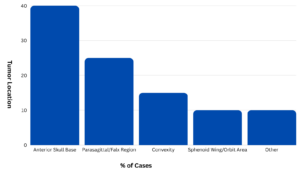
However, the research revealed a significant increased risk of meningioma with prolonged use of three specific progestogens: medrogestone (odds ratio 3.49), medroxyprogesterone acetate injection (odds ratio 5.55), and promegestone (odds ratio 2.39). The risk was particularly pronounced with prolonged use (more than one year) compared to short-term use, and the meningiomas were most commonly located at the base of the skull or the convexity. No malignant meningiomas were found among cases exposed to these three progestogens.
The study’s findings have particular relevance for global health, especially regarding medroxyprogesterone acetate, the active ingredient in Depo-Provera, which is widely used as injectable contraception worldwide and found to have a 5.6-fold increased risk in causing these types of brain tumors. Despite this, Pfizer has still not issued any warnings and the FDA has not required any type of black box warning like it had for decreased bone loss.
Intracranial meningiomas are tumors that develop in the meninges, the protective membranes covering the brain and spinal cord. These tumors originate from arachnoid cap cells, which are found in the middle layer of the meninges called the arachnoid mater. While most meningiomas (approximately 90%) are benign (WHO Grade I), they can still cause significant symptoms due to their location and the pressure they exert on surrounding brain tissue.
Risk factors for developing meningiomas include female gender (women are about twice as likely to develop them), advancing age, previous radiation exposure to the head, and certain genetic conditions like neurofibromatosis type 2. The female predominance suggests a hormonal influence, as many meningiomas have progesterone receptors. This is a possible key to why Depo-Provera can cause brain tumors and which is why any victims with this type of brain tumor should contact our Depo-Provera injury lawyer in Kentucky at the Maze Law Offices.
Symptoms of intracranial meningiomas vary depending on their location, size, and number of the because some victims using this birth control shot have even had multiple tumors.
Common presentations of an intracranial meningioma include the following:
Some meningiomas are discovered incidentally during brain imaging for unrelated conditions, and many grow so slowly that they may not cause symptoms for years. The growth rate varies, with some tumors remaining stable in size while others grow more rapidly. With some of the research from the BMJ, it appears some tumors have grown quickly from the active ingredients in Depo-Provera.
Diagnosis typically involves neuroimaging studies and other diagnostic studies. Sometimes this includes multiple different types of objective tests, from blood tests to imaging. As a result, it can also involve many different types of medical providers and specialists.
Some of the most common testing includes the following:
Once diagnosed, treatment options depend on various factors including tumor size, location, patient age, patient overall health, severity of symptoms, growth trends, risks, future risks and possibilities, patient preferences, and other treatment needs.
When an intracranial meningioma has been diagnosed, or if multiples, the care and treatment of a patient depends on many different factors as noted above. Once considered, the main types of treatment for a patient with this type of brain tumor from Depo-Provera or other birth control shots could be the following:
The prognosis for most patients with meningiomas is favorable, with five-year survival rates exceeding 90%-95% for benign tumors and over 60% for high grade and advanced malignant brain tumors. However, regular follow-up is essential as recurrence can occur even years after treatment. The recurrence rate varies from 7-20% for totally resected benign meningiomas to higher rates for subtotally resected or higher-grade tumors.
Recent advances in treatment include targeted therapies for recurring or aggressive cases, improved surgical techniques, and better radiation delivery systems. Ongoing research focuses on understanding the molecular and genetic factors driving meningioma growth, which may lead to more personalized treatment approaches in the future.
Understanding the classification of meningiomas is crucial for treatment planning and prognosis. There are different types that our experienced Depo shot lawsuit attorneys in Kentucky knows could be diagnosed due to these birth control shots. As a result, diagnosing and identifying the type can be important for your personal injury lawsuit or class action lawsuit in order to prove damages.
They include the following grades or classifications:
Intracranial meningiomas represent a complex medical condition requiring careful attention from both medical and legal professionals – especially as state and federal lawsuits involving Depo-Provera begin to start and gain more and more traction. Understanding the various aspects of these tumors—from diagnosis through treatment and recovery—is essential for providing comprehensive support to affected individuals, which means following up and following all care from your treating physicians.
For patients and families affected by intracranial meningiomas, having knowledgeable legal representation can make a significant difference in navigating the complex healthcare system and securing necessary resources for treatment and recovery. That means having an experienced personal injury lawyer like our knowledgeable Depo shot lawsuit lawyers to help you and your family.
In addition to brain tumors, there are other brain injuries that could be caused by Depo-Provera. These have been alleged in the various lawsuits, including some of the more recent ones that have been filed post-BMJ study. These can be symptoms of a brain tumor, but they can also be their own, independent conditions that are due to failed warnings on the birth control shots.
Some of these other types of injuries that could be related to brain tumors or could be another type of injury that should be evaluated by our Depo shot lawyers include the following:
Chronic headaches are defined as head pain that occurs 15 or more days per month for at least three consecutive months. Unlike occasional headaches, chronic headaches significantly impact daily life and may indicate underlying health conditions. Many chronic headaches are also classified as long headaches, which are episodes that last for four or more hours at a time. Brain tumors from Depo-Provera could cause headaches, including chronic or long headaches. They could also be caused independently as a symptom due to the active ingredients.
There are many possible signs of chronic headaches due to birth control injectables, but some of the most common include the following:
Treatment for chronic headaches typically requires a comprehensive approach that combines multiple strategies, which usually starts with stopping to take the birth control shot. Healthcare providers often start by prescribing preventive medications such as beta-blockers or anti-seizure drugs to reduce the frequency of headaches. For acute attacks, pain-relieving medications can provide temporary relief. Lifestyle modifications play a crucial role in management, including stress reduction techniques, maintaining regular sleep patterns, and identifying specific triggers to avoid.
Many patients find relief through alternative therapies such as acupuncture or biofeedback, which can complement traditional medical treatments. While complete elimination of chronic headaches may not always be possible, most patients can achieve significant improvement in both frequency and severity of their symptoms with proper medical care and lifestyle adjustments. Long-term success often depends on patient commitment to treatment plans and regular follow-up with healthcare providers. This is also important because it can be used to help support a medical malpractice, class action, product liability, failure to warn, or another type of lawsuit against a drug provider or manufacturer like Pfizer for Depo-Provera.
Epilepsy is a neurological disorder characterized by recurrent, unprovoked seizures due to sudden bursts of electrical activity in the brain. It affects people of all ages and can significantly impact quality of life if not properly managed. It is commonly referred to as a seizure disorder or chronic brain disorder, and it can be caused by Depo-Provera by itself or as a symptom of the brain tumors caused by the active ingredient. Thus, any person diagnosed with epilepsy after taking Depo-Provera should call our Depo-Provera injury lawyer in Kentucky at the Maze Law Offices for help.
There are many signs of epilepsy, and although the main symptoms might appear to be obvious, that is not always the case. Indeed, some seizures are not as obvious or expected, especially for people who do not have a history of seizures before taking the depo birth control shot.
Some of the most common symptoms include the following:
Treatment for epilepsy due to brain tumors focuses primarily on controlling seizures through medication. Anti-epileptic drugs successfully control seizures in many patients, though finding the right medication and dosage may take time. For some individuals, particularly those who don’t respond well to medications, doctors might recommend dietary changes such as the ketogenic diet, which has shown promising results in reducing seizure frequency. Of course, one of the more obvious choices would be to also treat the brain tumor itself by removing it. However, that is not always the cause of epilepsy, and may only be a symptom. The Depo birth control shot could cause this by itself, without brain tumors, as we are still learning more about the dangerous side effects that Pfizer may not have totally warned us all about.
In cases where medications and dietary changes prove insufficient, surgical intervention might be considered. Modern surgical techniques, combined with careful patient selection, have shown excellent results in reducing or eliminating seizures in appropriate candidates. Additionally, devices like vagus nerve stimulators can provide significant relief for some patients. The overall prognosis for epilepsy is increasingly positive, with approximately 70% of patients achieving seizure freedom through various treatments. Many children may eventually outgrow their epilepsy, and adults can often lead normal lives with proper medication and lifestyle management.
Vision loss refers to partial or complete loss of vision that cannot be corrected with standard eyeglasses or contact lenses. It can occur gradually or suddenly and may affect one or both eyes. This is particularly true as the brain tumors from the birth control shots are still growing, or growing slowly, and increase in side and place pressure on the brain. That’s why our Depo-Provera injury lawyer wants victims and their families to always ask for help and determine whether their vision loss was caused by this drug or not. The damages are severe and can be very serious.
There are many signs of vision loss due to brain tumors from the birth control shots. Some signs are obvious, like loss of vision, but other signs like loss of peripheral vision can be tricky and harder to determine.
Some of the most common symptoms include the following:
If you have any of these troubling symptoms after taking the Depo-Provera birth control shot – even if you just had it once – call the Maze Law Offices to learn more about your rights. Vision loss could be due to a brain tumor, or it could be by itself due to the caustic nature of the active ingredients.
The treatment approach for vision loss varies significantly depending on the underlying cause, especially for a brain tumor like intracranial meningiomas. For conditions like glaucoma, regular use of prescribed eye drops and medications can help prevent further vision loss, while cataracts can often be successfully treated with surgery, resulting in dramatic improvement in vision. Retinal conditions may require specialized treatments ranging from laser therapy to surgical intervention.
Vision rehabilitation programs play a crucial role in helping patients adapt to vision loss, teaching valuable skills and strategies for maintaining independence. Modern assistive technologies have revolutionized the way people with vision loss can interact with their environment and continue daily activities. The prognosis varies considerably based on the specific condition – some causes of vision loss are completely reversible with treatment, while others may result in permanent changes. However, even in cases of significant vision loss, proper support and rehabilitation can help patients maintain a high quality of life – especially from brain tumors like the depo birth control shots. The key to optimal outcomes lies in early detection and prompt treatment, making regular eye examinations essential, particularly for those at higher risk of eye diseases.
The Maze Law Offices handles personal injury cases involving the birth control shot Depo-Provera (medroxyprogesterone acetate), an injectable contraceptive manufactured by Pfizer, which has become the subject of notable product liability litigation. This is an emerging issue in law and the cases are being filed right now, meaning victims need to contact our experienced Depo Shot Lawyers in Kentucky right now to be included and not left behind.
In these cases, the plaintiffs have been alleging links between the medication and the development of brain tumors. This is supported by the March 2024 BMJ study and publication that there is a 560% increase in the occurrence of brain tumors in patients who have taken these shots.
The top three of the most important and key product liability claims that are being alleged against Pfizer and the owners, manufacturers, retailers, prescribers, makers, marketers, and other defendants profiting or otherwise advancing this drug include the following:
The central allegation in many lawsuits is that Pfizer failed to adequately warn healthcare providers and patients about the potential risk of brain tumors associated with Depo-Provera use. As you may recall, Pfizer may have done this before with the bone loss lawsuit in Canada and the allegations there.
This type of allegation includes the following claims:
Plaintiffs have alleged that Depo-Provera’s design posed unnecessary risks to users. Key claims include design defects in the drug and the product that have increased the risk and dangers for users. Some of these claims include the following:
The manufacturing defect claims focus on several errors in the way that the birth control shot was made. Meaning that they are all defective and dangerous within the same lot. Some of these allegations include the following:
A victim who has a brain tumor, chronic headaches, epilepsy, loss of vision, or any other damages related to this type of birth control shot may be entitled to “damages” with the help of a Depo-Provera injury lawyer like ours at the Maze Law Offices. Under Kentucky law and federal law, the term “damages” is the measure of relief that a court could grant a party in a lawsuit. That includes as an individual or in a class action lawsuit or mass tort lawsuit.
In personal injury lawsuits, like cases based on negligence and products liability such as for a dangerous birth control shot, victims are typically entitled to damages which are in the form of monetary compensation. There are many different types of monetary compensation that a victim could be awarded, which include the following:
If you or your loved one were injured by a birth control shot, call the Maze Law Offices for help today. Medical research has confirmed a link between the active ingredient in Depo-Provera and brain tumors, and victims may be entitled to compensation for their pain and suffering, lost wages, medical bills, and other damages relating to this injectable. Unfortunately, Pfizer failed to warn patients of bone loss which was a part of another lawsuit that resulted in a multi-million dollar settlement, and early indications here show that they may have failed to warn patients of the increased risk of brain tumors as well.
Whether you had one shot or were on the shot for many years, anyone who was diagnosed with a brain tumor, intracranial meningioma, epilepsy, chronic headaches, vision loss, any type of cancer, or any other conditions that may have been caused by this birth control shot, call our Depo-Provera lawsuit attorneys in Kentucky to schedule a free consultation. All consultations are free and there are no upfront costs to begin with our law firm because we pay all the litigation expenses for you, which are only reimbursed once we recover compensation for you. The same is true of our legal fees, which only come from a percentage of what we recover for you in a verdict, settlement, or another type of award.
Schedule Your FREE Case Evaluation to Have Your Depo Shot Questions Answered
To get started with our experienced Depo-Provera injury law firm, schedule your free consultation and case evaluation by sending us a confidential message through our “contact us” box available here or calling us by dialing (859) 900-9000. There is no fee unless we win for you and your family. Given the gravity of a catastrophic brain tumor case and the fact that other plaintiffs are actively commencing lawsuits throughout the country, do not delay in calling us to schedule your free case evaluation – call now to protect your rights and obtain the compensation that you deserve and need to move forward.
 N/a
(859) 882-9999
N/a
(859) 882-9999